Abstract
Introduction: Pulmonary embolism (PE) is a common complication of cancer and its treatments; it is a leading cause of morbidity and mortality in patients with malignancy. While extensive work has been done in evaluating PE risk of patients with cancer, there are limited data on the prognostication of adverse outcomes when these patients do experience an event. Guidelines recommend the use of clinical prediction rules to help clinicians identify patients at low- and higher-risk for adverse outcomes and guide the appropriate level of care. However, no single clinical prediction rule has been shown to be superior in stratifying these patients. Thus, we sought to determine the accuracy of clinical prediction rules for identifying patients with cancer and acute PE at low risk for mortality.
Methods: A systematic literature search of Medline and Scopus from January 1, 2000 through July 1, 2017 was performed, along with a manual search of references. Studies deriving/validating at least one clinical prediction rule for early post-PE all-cause mortality in patients with cancer and providing mortality data over at least the index PE hospitalization but not longer than 90 days were included. Only full-text, English-language articles were included. A bivariate model was used to pool sensitivity and specificity estimates for each clinical prediction rule using a random-effects approach. Traditional random effects meta-analysis was performed to estimate the weighted proportion of patients deemed at low risk for early mortality and their odds ratios for death compared with high-risk patients. Statistical heterogeneity was assessed with the I2 statistic with values >50% deemed as high.
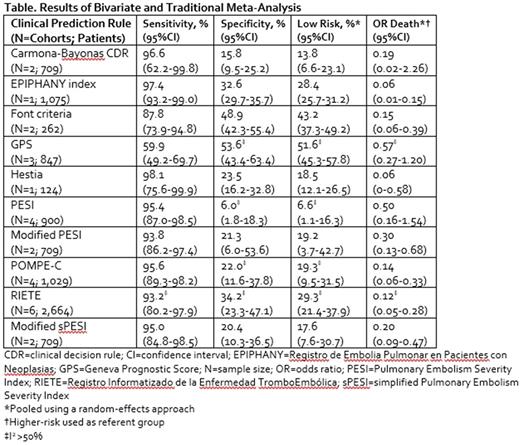
Results: We identified 1,104 non-duplicate citations from our literature search. Eight studies evaluating ten clinical prediction rules in 3,974 (range 124 to 1,075) unique patients were ultimately included in our meta-analysis (Table). Mean/median patient age ranged from 59 to 71 years. The majority of included patients were receiving chemotherapy and/or radiotherapy (50 to 75% of patients), and metastatic disease was observed in 49% to 74% of patients. The Hestia and EPIPHANY index rules displayed the highest sensitivities (98.1% [95% CI = 75.6% to 99.9%] and 97.4% [95% CI = 93.2% to 99.0%], respectively) with the remaining rules' sensitivities ranging from 0.88 to 0.97; only the GPS tool had a sensitivity of 0.60. Of the six clinical prediction rules with sensitivities ≥95%, none had specificities >33%. Traditional random-effects meta-analysis suggested anywhere from 6.6% to 51.6% of cancer patients with PE were at low risk and that low-risk patients had a 43% to 94% lower odds of death compared with those at high risk.
Conclusions: There is a limited total body of evidence on the ten clinical prediction rules included in our meta-analysis. As such, the results of clinical prediction rules as well as other patient-specific clinical factors should continue to be used in identifying appropriate treatment for patients with cancer experiencing an acute PE.
Coleman: Bayer AG: Consultancy, Honoraria, Research Funding; Janssen Pharmaceuticals: Consultancy, Honoraria, Research Funding; Boehringer Ingelheim: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal